Quality Systems
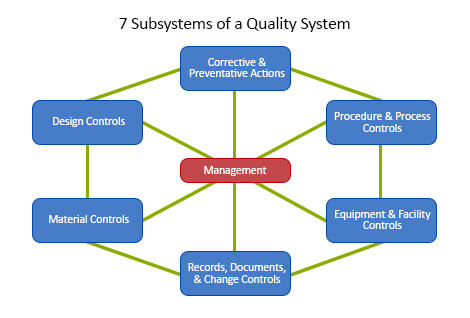
The FDA defines a Quality System as the organizational structure, responsibilities, procedures, processes, and resources for implementing quality management.
Each manufacturer is expected to develop a Quality System that is commensurate with the risk presented by the product, the complexity of the product and manufacturing process, and the size and complexity of the manufacturing facility. The bottom line is that there is no such thing as a one-size-fits-all Quality System.

Our goal is to satisfy your business and Quality System requirements to ensure your product remains safe and effective and stays on the market. Our experience includes compliance with the FDA Quality System Regulation, 21 CFR 820, ISO 13485:2016, and EU MDR.

We offer scalable support based on your risk and specific QS needs. Whether you need strategic assistance responding to an FDA enforcement action (Form 483, Warning Letter, Consent Decree), ISO Certification, full assessment of your Quality System (CAPA, Complaint Handling, Audits, Design Controls, etc.), tactical remediation of a problem area, or project team staff augmentation.
Quality Systems Assessment
- Compliance Risk Assessment
- Gap Assessment
- Company Culture Assessment
- Enforcement Action Response
- Remedial and Strategic Solutions
- Develop Whole System Approach
- Proactively Communicate with FDA/Notified Body

Strategic Project Partner
Raland is a strategic project partner with QRx Partners. Together, we leverage our combined strength and expertise to deliver superior consulting services for select client projects. Check them out!