Computer Systems Validation

Your credibility depends on meeting the guidelines set forth by the Code of Federal Regulations Section 21, Part 11 (21 CFR Part 11) and EU GMP Annex 11 for electronic records and electronic signatures (ERES). Raland Compliance Partners understands global requirements and industry regulations. We work to help you meet and exceed them.

FDA guidance defines software validation as “confirmation by examination and provision of objective evidence that software specifications conform to user needs and intended uses, and that the particular requirements implemented through software can be consistently fulfilled”. Our CSV professionals can support various SDLC methodologies with Waterfall and Agile being the most common for life science companies. We are fluent in the full suite of SDLC deliverables from Requirement Specifications (User, Functional, and Design) through IQ, OQ, and PQ protocols and full traceability.
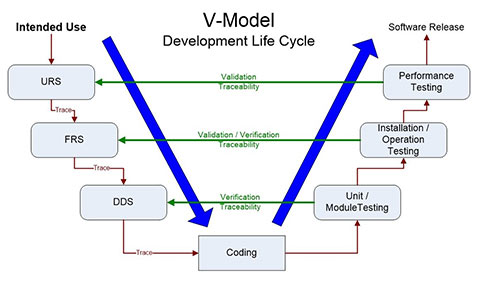
We'll develop a customized plan for your project, aligned with your risk tolerance, internal processes and procedures, leveraging accepted vendor documentation to assure that your system meets your user requirements and intended use.
Our CSV practice includes the following services:
- IT-Based Software Validation
- Building Management Systems Validation
- Laboratory Information Systems Validation
- Laboratory Instrument Qualification
- Quality Management Systems Validation
- Electronic Document Management Systems Validation
- ERP/MRP Systems Validation
- Data Centers and IT Infrastructure Validation
- Software as a Service (SaaS) Validation
- Software as a Medical Device (SaMD) Validation